There are many areas in which biotechnology is applied. Biotechnology is being used in all aspects of human life, from food to agriculture/forestry and fisheries, the environment, energy, and chemicals. Current biotechnology is represented by genetic modification technology. Such technology entails safety issues for humans and the environment.
In order to secure safety for LMOs which are the highest-level product of Korea’s modern biotechnology, we have two laws in place. First is the Act on Transboundary Movement of LMOs. The other is the Food Sanitation Act.
The LMO Act is an act for the implementation of the Cartagena Protocol on Biosafety. Its purpose is to assess the risk of LMOs, and manage risk in order to secure safety. The Food Sanitation Act consists of safety management and the mandatory labeling system for genetically modified food, as well as human health risk assessment and review of LMOs used as food.
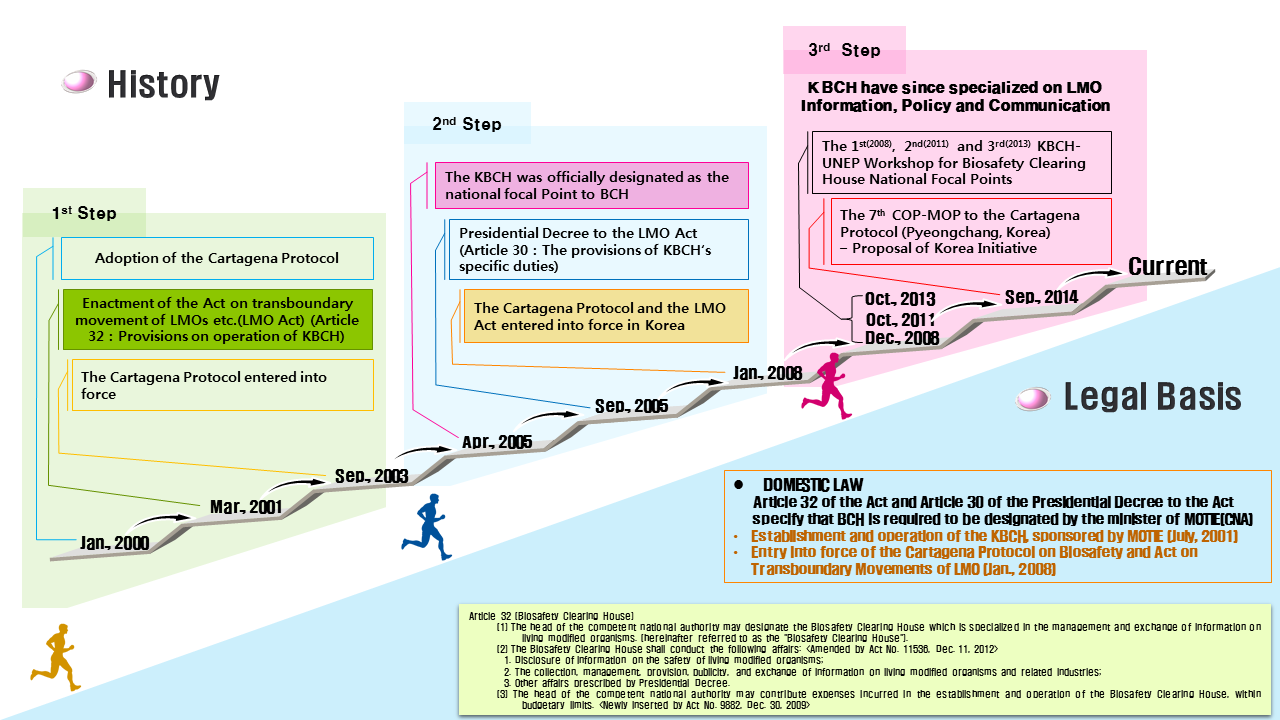
The international community has strived to attain sustainable preservation of biodiversity and protection of human health, pursuant to the use of LMOs, through the Cartegena Protocol. Consequently, based on the Convention on Biological Diversity, the Cartagena Protocol on Biosafety came into effect in 2003. This Protocol contains prescriptions on the following: the precautionary principle; advanced informed agreement; risk assessment; risk management and emergency measures; treatment and transportation, packaging and labeling; Biosafety Clearing House; and public awareness. In order to implement this Protocol, Korea announced the LMO Act in 2001, which came into effect in January of 2008.
The Protocol recommends the provision of laws in each country for its implementation. In addition, it prescribes that a competent national authority and an entity that can share LMO related information be in place. In response to these provisions, Korea has enacted and is implementing the LMO Act, with the Ministry of Trade, Industry and Energy as the competent national authority. As the information sharing entity, KBCH has been legally designated.
Korea also has an agency that is responsible for safety management, which is broken down according to purpose of usage of the different types of LMOs. R&D purpose LMOs are managed by the Ministry of Science, ICT and Future Planning; LMOs for agriculture and livestock breeding purposes are managed by the Ministry of Agriculture, Food and Rural Affairs. Industrial purpose LMOs are managed by the Ministry of Trade, Industry and Energy; Health and medical purpose LMOs are managed by the Ministry of Health and Welfare. LMOs for environmental purification purposes are managed by the Ministry of Environment. Maritime and fisheries related LMOs are managed by the Ministry of Oceans and Fisheries; LMOs for food and medical devices are managed by the Ministry of Food and Drug Safety. In regard to all matters for Protocol implementation and LMO safety management, the Biosafety Committee is the deliberating agency that oversees all related tasks.
The life cycle of an LMO begins with Research & Development. The following is about the development trend of GMO in Korea. The most representative is the development of fluorescent fish, GM rice, and fluorescent silkworms. One of the Korean universities has completed fluorescent fish development and is currently in the risk assessment stage. After completing the risk assessment, it will apply for risk review.
Korea's Rural Development Administration has also completed the development of functional GMO rice and fluorescent silk silkworms. The RDA is currently preparing for the risk assessment.
In Korea, such LMO research and development is proceeding very actively.
R&D must be conducted in a closed research facility.
Each LMO subject to research and development is included in a Risk Group. When developing organisms that do not pose any risk to the human body or the environment, or using them for tests, the work is conducted in a Level 1 research facility. Sometimes the work can result in fatal illnesses to humans, regarding which treatment is difficult. When released into the environment, the damage is considerable and recovery is not easy. When developing LMOs or using them for tests that have such potential effects, the work must be conducted in a Level 4 research facility.
Level 1 and 2 national research facilities must be reported to the Ministry of Science, ICT and Future Planning. Level 3 and 4 facilities must receive a permit from the Center for Disease Control. Development and tests can be conducted freely in national research facilities that have been registered officially through reports or attainment of permits. However, in the case of developments or tests that are acknowledged as being a risk to the human body, approval must be acquired from the government.
Persons or entities operating a research facility must receive regular inspections from the government. Each year, personnel must receive training regarding safety management for a certain period.
Currently, Korea imports a great deal of GM crops for food and feed. In 2014, the import amount exceeded 100 million tonnes for the first time. The most imported crop was corn, followed by beans. Korea is not yet growing LMOs.
In Korea's LMO legislation, it is necessary to take a risk review before importing LMO.
LMOs for food are reviewed by the Ministry of Food and Drug Safety, while LMOs for feed are reviewed by the Rural Development Administration, which is an agency under the Ministry of Agriculture.
The risk assessment procedure is unique to Korea, and is a joint review system. Under this system, several risk review agencies that specialize in a particular type of LMO conduct a review on the pertinent subject, and share the results. The system attains safety more successfully than almost any other country in the world.
The following is a description of the system introduced and enforced for the first time through the revision of the LMO Act in 2012. It is the safety management system for LMM(Living Modified Micro-organism). In Korea, in order to use LMM, the requirement is to use a facility for contained use. Level 1 and 2 facilities must be reported, while Level 3 and 4 facilities must receive a permit. In case of non-compliance, punitive actions imposed include imprisonment, fines or penalties.
However, when it is judged that the LMM the producer intends to use has no risk at all, an application can be made for a simplified risk assessment procedure for LMMs to be used in a Level 1 Facility for Contained Use.
In Korea, the general risk review period is 270 days. However, for LMMs used in Level 1 Facilities for Contained Use, the risk review period is reduced to 90 days.
Currently, we have been two cases experience of risk review.
First is being conducted for the callus of rice, which is being developed as a human epidermal growth factor. Second is LM E.coli, which is developed as a enzyme for production of raw material detergent. They are being treated as an LMO for industrial use and the condition is contained use. Government approval is required for use of LMM.
To apply for approval of usage, two conditions must be satisfied. The facility should be contained by related administrative agencies, and the subject LMM must have completed risk review. To apply for usage approval, one must submit the standard manual for treatment and operation of the facility, a list of biosafety experts necessary for safety management, and the current status and condition of facilities.
Such data is reviewed by the related central administrative agency, and a final decision is made on whether approval shall be given. LMOs that have completed the risk review can be imported, produced and used.
However, this also requires government approval. The purpose of this approval procedure is to confirm through documents that the LMO that is intended for import and production is the very LMO that received the risk review. Thereafter, when the LMO actually enters Korea, an inspection is made to check that it is the LMO for which a review was completed and approval was acquired.
LMO safety management is required to prevent possible risks in the distribution and consumption stages, even if the LMOs are identified as safe. The national entity is directly involved in monitoring to see if a mechanism has been installed in transportation vehicles in order to prevent non-intended release when transporting, and to check for any instances of unintended release.
Now the last paragraph. It is the final stage for LMOs – consumption.
The major principle of LMO safety management is to prohibit use of LMOs that are unsafe. The principle is that only LMOs and GMO products regarding which safety has been confirmed can be delivered to the consumer.
Despite, this, Korea is implementing the labeling system for consumers’ right to know.
However, in response to this, consumers sometimes run anti-GMO campaigns demanding a complete labeling system and history tracking system.
For the implementation of the Biosafety Protocol, KBCH provides accurate and timely information regarding LMOs to the public.
KBCH cooperates with 7 national agencies that work for national LMO safety management.
Its role is to improve and consolidate Korea’s LMO safety management law and system. It strives to provide balanced information regarding the safety of LMOs to developers, producers, distributors and consumers alike. Information sessions regarding the LMO Act, debate tournaments for high school students on the subject of LMOs, presentations regarding LMOs for housewives, a model UN assembly meeting held last year, LMO forums and seminars are the many events that we sponsor to approach the public.




























