The Philippines ratified on the Cartagena Protocol on Biosafety on August 14, 2006 and entered into force on January 8, 2007. Prior to that, the Philippines already had an extensive experience on regulating Genetically Modified Organisms (GMOs).
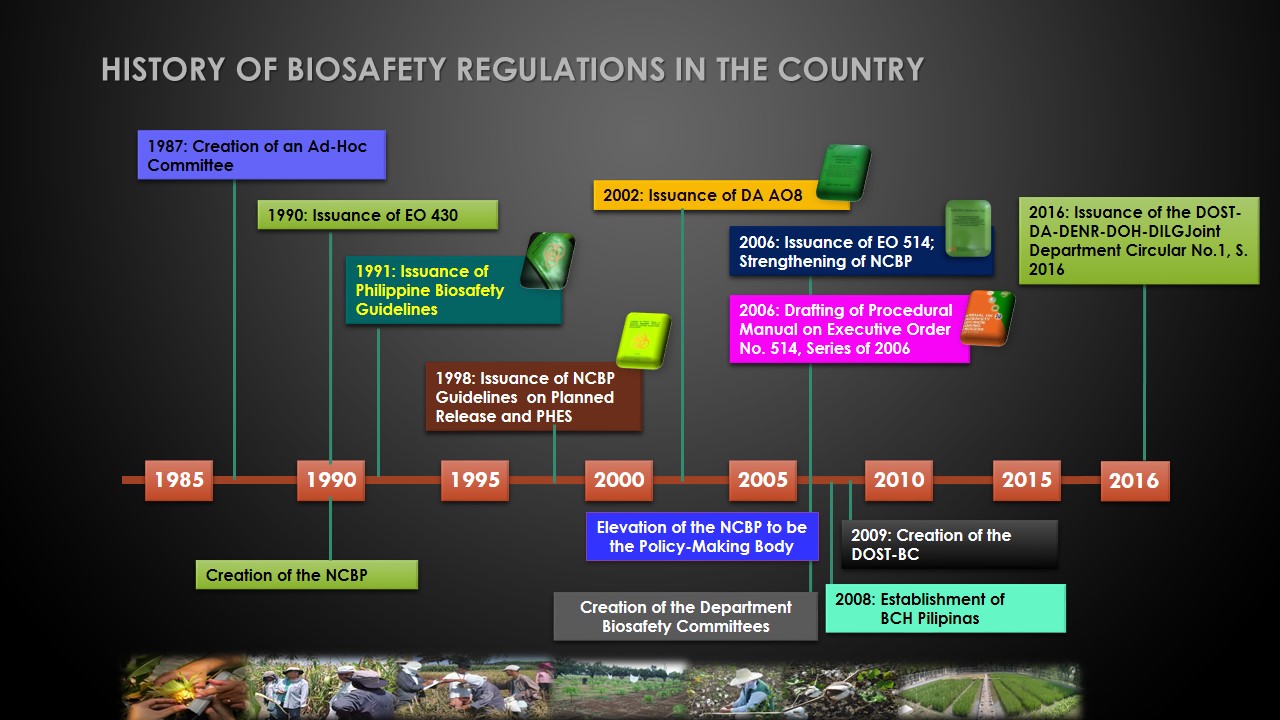
The Philippines is the first country in Asia to establish its biosafety regulatory system for GMOs. The initiative to establish a a biosafety framework cam from scientists in 1987. The biosafety regulatory system of the country was based on Executive issuances and is not legislated. It is also compliant with the international standards and obligations.
The Biosafety assessments is done by the Competent National Authorities of the government, International Biosafety Committee (IBC) as well as the scientists. These assessments done in the Philippines are science-bases and is done on a case to case basis (depending on the utility of the GMOs).
Interest in biosafety started when the University of the Philippines - Los Baños (UPLB) and the International Rice Research Institute (IRRI) required the use of modern biotechnology tools. Hence, the scientists raised their issues of concerns. In 1987, the Philippines created a Joint Committee to study the need for formulation of guidelines for Research and Development. The Committee was composed of scientists from UPLB, IRRI, Philippine Council for Agriculture, Forestry and Natural Resources Research and Development (PCARRD) and Plant Quarantine Services. The said guidelines were presented by the National Academy of Science and Technology (NAST) during a nation-wide consultations. It is recommended that the guidelines be adopted nationally and a body be created to serve as oversight for biotech research activities.
The Executive Order 430 was created in 1990 which led to the creation of the National Committee on Biosafety of the Philippines (NCBP). The functions of which are the following:
- Functions both as a policy making and implementing body
- Formulate policies and issues guidelines on biosafety
- Identify potential hazards involved in genetic engineering experiments
- Prescribe measures to minimize the risk on the proposed activities
- Serve as oversight for genetic engineering experiments, thru the IBCs
- Develop working arrangements with quarantine services for compliance with the quarantine requirements and importation of GMOs used in research
The following the E.O. 430, guidelines were created in order to regulate GMOs:
1991:
Philippine Biosafety Guidelines - contained use and field testing of GMOs; application procedures, containment requirements and safety evaluation of proposals
1998:
Planned release guidelines for GMOs and Potentially Harmful Exotic Species (PHES)
2002:
1) A.O. 8; Transferred the decision-making process and monitoring of field testing and commercial propagation to the Department of Agriculture - Bureau of Plant Industry (DA-BPI); Served to formalize existing arrangements between NCBP and DA– that review and monitoring for contained experiment shall remain with the NCBP; Spells out the rules and procedures for the importation and release into the environment of plants and plant products derived from the use of modern biotechnology
2) A.O. 2 Handling, importation, field trials and commercial propagation of GM crops
2006:
E.O. 514 National Biosafety Framework; The NCBP, from regulatory body, became a policy making body and it expanded its membership to include other Departments and other sectors of society; Delineates the responsibilities of regulatory agencies involved in GMO regulations with regard Risk Assessment; Preparatory to extended genetic engineering activities not only crop plants but also livestock, microorganisms , pharmaceuticals, nutraceuticals, bioremediation agents and other types of GMOs
2014:
1) Revised Guidelines on Contained Use of GMOs as per E.O. 514;
2) Guidelines for Contained Use of GM Arthropods
2016:
DOST-DA-DENR-DOH-DILG Joint Department Circular; R&D, importation, handling and transport, F.T. and commercial propagation and direct use of GMOs




























